Chromatography is a fascinating scientific technique used to separate and analyze mixtures of substances. Many things around us, from the ink in our pens to the medicines we take, are actually made up of mixtures. Chromatography helps us “unlock” these mixtures, showing us their hidden components by separating them based on how they move through a material such as paper or gel.
In this activity, you will use paper chromatography to uncover the hidden pigments inside ordinary colored marker pens. By placing the ink on filter paper and letting water carry it upward, you will see how what looks like a single color is actually made up of several different dyes. Each pen will reveal its own unique “fingerprint” of colors, turning science into both an experiment and a work of art!
Science Involved
The basic idea behind chromatography is that different substances travel at different speeds through a medium when carried by a liquid (called the solvent). For example, if we place a small spot of ink on a strip of paper and dip it into water or alcohol, the liquid slowly moves upward through the paper. As it rises, it pulls the pigments in the ink along with it. Because each pigment has a unique chemical structure and interacts differently with paper and the solvent, some pigments move faster and travel farther, while others move more slowly and stay near the starting point. The result is a beautiful separation of colors that reveals the “hidden recipe” of the ink.
Applications of chromatography
Chromatography isn’t just a cool experiment in the lab – it has many interesting and important real-world uses. Here are a few fascinating applications:
-
Crime Investigations: In forensic science, chromatography is used to analyze evidence like ink from documents or substances found at crime scenes.
-
Food Testing: Chromatography is used to check for additives, artificial colors, and contaminants in food. It helps ensure that what we eat is safe and healthy by identifying harmful chemicals or ensuring that the correct ingredients are used.
-
Medicine Development: In the pharmaceutical industry, chromatography is used to test and purify medicines. It helps scientists separate and study different chemical compounds, ensuring that the drugs are pure and work effectively.
-
Environmental Testing: Chromatography is used to test water, air, and soil for pollutants and toxins. Scientists can identify and measure harmful substances, which can help in protecting the environment and keeping people safe.
-
Sports and Doping Tests: In sports, chromatography is used to detect banned substances in athletes' blood or urine samples. This ensures fair play by revealing whether someone has used performance-enhancing drugs.
From catching criminals to protecting our health and environment, chromatography plays a role in solving real-world mysteries every day.
Activity
Requirements
250 mL beaker, solvent (water), filter paper, colored markers (black, red, green, yellow, blue, orange), pencil, ruler, tape, plate or dish (for drying) and scissors.
Procedure
-
Cut the filter paper into six strips, each measuring 1 cm by 10 cm.
-
On each strip, draw a horizontal line 2 cm from the bottom and another at 6 cm from the same end using a pencil. These will serve as the starting line and the maximum solvent front for the chromatography process.
-
Using a black marker, place a small dot on the strip 2 cm from one end (at the starting line). Repeat for each color: red, green, yellow, blue, and orange, making sure to place the dot at the 2 cm mark on each strip.
-
Tape up to three strips side by side to a pencil, making sure the strips are evenly spaced and do not touch each other.
-
Pour 10 mL of water into the glass beaker.
-
Lay the pencil across the rim of the beaker so that the strips hang into the beaker and their ends just touch the water (solvent), ensuring that the dots are above the solvent level.
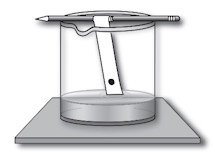
Paper chromatography setup. Image Credit: Cameron, Schyrlet. "Petty Larceny". Forensic Investigations. Mark Twain Media Inc. 2008. -
Allow the solvent to travel up the strips through capillary action. Observe as the solvent interacts with the marker dots, separating the pigments.
-
Once the solvent reaches the 6 cm line, carefully remove the strips from the beaker and place them on a plate to dry.
-
After the strips have dried, tape them in the observation table.
-
If needed, repeat the process for any remaining strips by attaching them to the pencil and following the same procedure.
-
After the strips have dried, select the one with the orange marker for analysis.
-
Measure the distance traveled by each pigment from the starting line and compare it to the distance traveled by the solvent.
-
Use these measurements to calculate the retention factor \((R_{f})\) for the pigments in the color.
Retention factor
Each separated band can be assigned a Retention Factor \((R_{f})\) which is characteristic of each specific dye(s). The \(R_{f}\) is a ratio of the distance the band travels to the distance the solvent (alcohol) travels. The \(R_{f}\) is calculated by dividing the band distance by the solvent distance. This ratio should be a constant that is characteristic of the dye(s) in a particular spot under a particular set of chromatographic conditions (i.e. paper chromatogram, alcohol solvent, etc.).
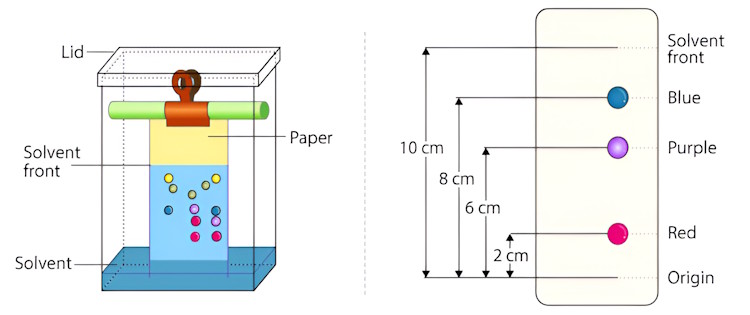
| $$R_{f} = \ \frac{Distance\ Traveled\ by\ Band}{Distance\ Traveled\ by\ Solvent}$$ | $$R_{f}(red) = \frac{2}{10} = 0.2\ and\ R_{f}(blue) = \frac{8}{10} = 0.8$$ |
|---|
Observations
| Color | Black | Red | Green | Yellow | Blue | Orange |
|---|---|---|---|---|---|---|
| Pigments identified |
Reflect and Discuss
-
Which pigment moved the most across all your strips?
-
Predict the pigments needed to create brown color.
-
Retention factor of all the pigments that are present in the orange marker.

