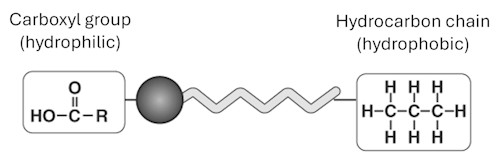
A molecule is so small that millions of them can fit into the head of a pin! Scientists in the early 20th century used clever experiments to figure out how big molecules are. In this activity, you will create an extremely thin layer of oil on water and use simple measurements to get an estimate of the size of a single molecule. It’s a little bit of math, a little bit of observation, and a lot of fun detective work in science.
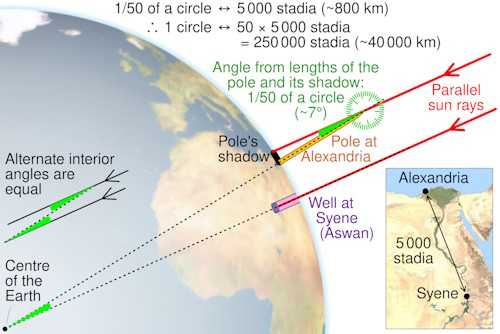
Over 2,000 years ago, a Greek scholar named Eratosthenes became the first person in history to calculate the circumference of the Earth. He lived in Alexandria, Egypt, and knew that in the city of Syene (now Aswan), the Sun shone directly into deep wells at noon during the summer solstice. By comparing the angle of the Sun’s rays in Alexandria with those in Syene, and knowing the distance between the two cities, he was able to estimate the size of the Earth with remarkable accuracy.
In this activity, you will follow a similar approach using maps and coordinates of Indian cities. You will measure distances, calculate angles, and estimate the Earth’s circumference, just like Eratosthenes did centuries ago.
Step into the shoes of an explorer mapping out unknown lands, just like the great explorers of the 15th and 16th centuries – think of Vasco da Gama or Christopher Columbus. Every step they took needed careful measurement; too short or too long, and their maps could lead ships astray.
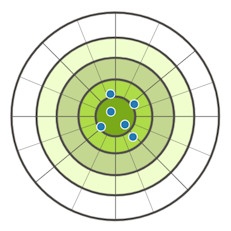
In science, we use two important ideas when measuring: accuracy and precision. Accuracy tells us how close a measurement is to the true value, while precision shows how close repeated measurements are to each other.
In this activity, you will test three different scales – one without markings, one perfectly marked, and one incorrectly marked – to see which ones help you be accurate and precise, just like a skilled explorer navigating the seas.

A candle holds secrets of chemistry, physics, and even the composition of air! Michael Faraday, one of the greatest scientists of his time, once gave a famous lecture series called The Chemical History of a Candle, where he explored how a simple flame reveals amazing scientific principles.
In this activity you will practice the scientific method by observing the candle and its flame under different conditions. As aspiring scientists, your most important tool right now is your observational ability. This activity is primarily about honing that skill. Since you're learning the scientific method, don't worry about getting all the "right" answers! Many of the explanations for what you observe are complex and will make more sense as you master higher-level science. For now, focus on seeing, recording, and questioning. Get ready to investigate this everyday object with new eyes – there’s a lot more science hidden in a candle than you might think!
For centuries Aristotelian philosophy dominated human thought. Scientific questions were answered through reasoning and debate, rather than through experiments. For example, it was believed that heavy bodies fall faster than light ones, but we have no record from those days of an attempt to study the motion of falling bodies.
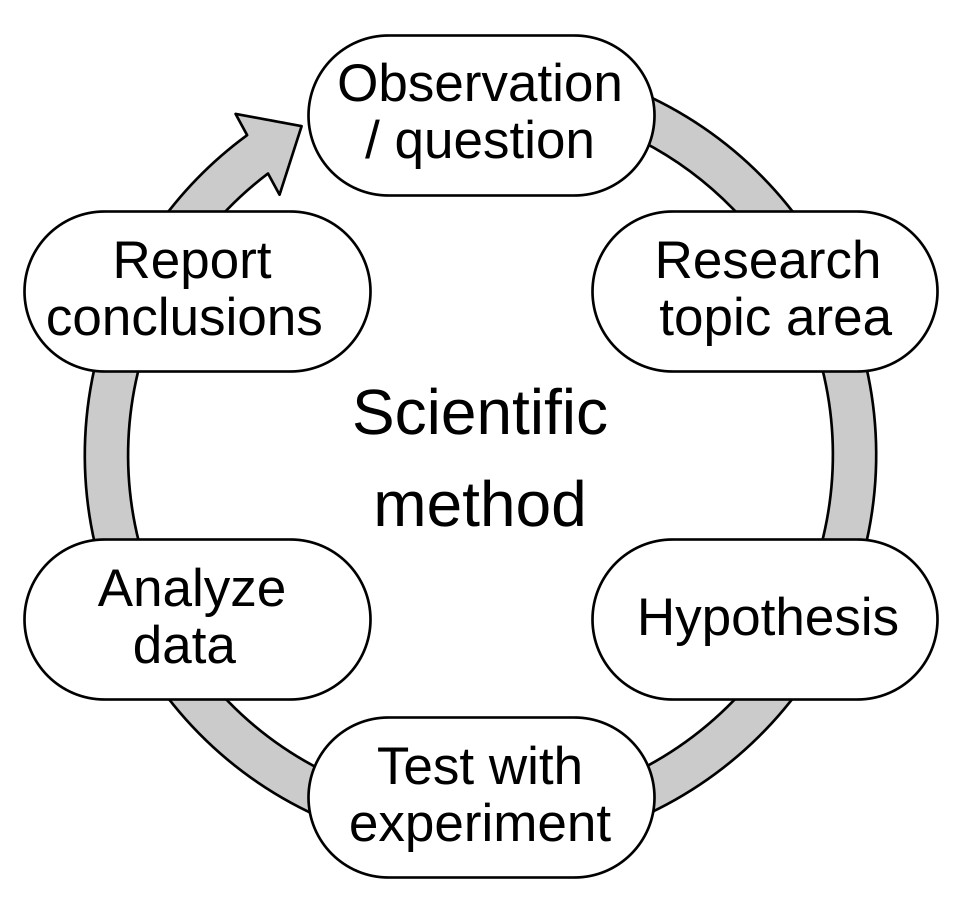
It was Galileo Galilei (1564 – 1642) who changed this way of thinking. He believed that nature’s truths must be discovered by observation and testing – not by argument alone. Through simple yet brilliant experiments, Galileo laid the foundation for what we now call the Scientific Method – a systematic way of asking questions, forming hypotheses, and testing them through evidence.
In this activity, you will learn how Galileo’s curiosity and experiments helped overturn long-held beliefs and opened the door to scientific thinking.