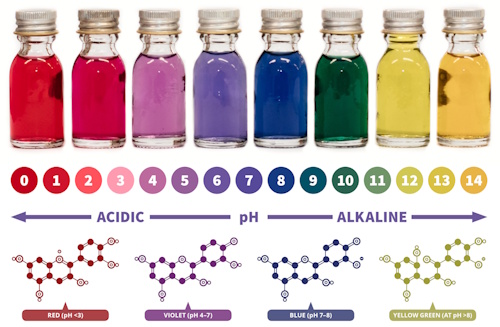
Chromatography is a fascinating scientific technique used to separate and analyze mixtures of substances. Many things around us, from the ink in our pens to the medicines we take, are actually made up of mixtures. Chromatography helps us “unlock” these mixtures, showing us their hidden components by separating them based on how they move through a material such as paper or gel.
In this activity, you will use paper chromatography to uncover the hidden pigments inside ordinary colored marker pens. By placing the ink on filter paper and letting water carry it upward, you will see how what looks like a single color is actually made up of several different dyes. Each pen will reveal its own unique “fingerprint” of colors, turning science into both an experiment and a work of art!