When your stomach feels uneasy after eating spicy food, what do you do? Many people take an antacid tablet. But did you know that when you do that, you’re actually performing a neutralization reaction inside your body?
Neutralization reactions have practical applications in daily life. From treating acidic soil on farms to making sure the water coming out of factories is safe for the environment.
In this activity, you will use two common laboratory chemicals – hydrochloric acid (HCl) and sodium hydroxide (NaOH) – to see how neutralization works. By measuring how much base is needed to completely neutralize the acid, you’ll also practice an important chemistry technique called titration.
Science Involved
Neutralization is a chemical reaction in which an acid reacts with a base to form salt and water. This reaction occurs when hydrogen ions (H⁺) from the acid combine with hydroxide ions (OH⁻) from the base to form water. The general equation for neutralization is:
Acid + Base → Salt + Water
Activity
Requirements
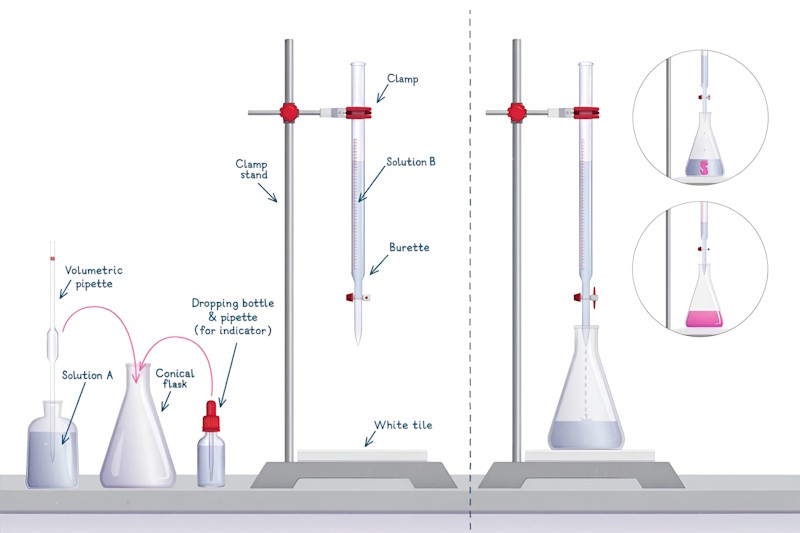
Burette and burette stand, conical flask, pipette (25 mL) or syringe, beaker, hydrochloric acid (HCl) solution of unknown concentration, sodium hydroxide (NaOH) solution of known concentration, phenolphthalein indicator and one white tile.
Safety Instructions
You will be working with acids and bases that can irritate your skin or eyes. Always wear safety goggles and a lab coat. Handle acids and bases carefully and never taste or touch them. If you spill any chemical, wash the area immediately with plenty of water and inform your teacher or parents.
Procedure
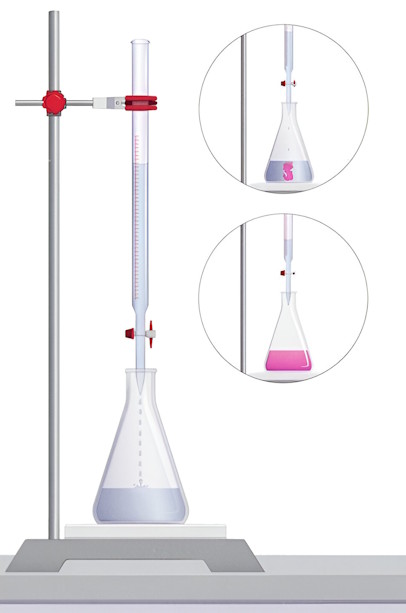
-
Rinse the burette with the base solution (NaOH) and fill it with the same solution using a funnel. Make sure there are no air bubbles. Record the initial reading.
-
Rinse the pipette with the acid solution (HCl) and use it to transfer 25 mL of HCl into a clean conical flask. Add 2-3 drops of phenolphthalein indicator to the flask.
-
Place the conical flask on a white tile to observe the color change clearly.
-
Slowly release the acid solution drop by drop from the burette into the conical flask while gently swirling the flask to mix the solutions.
-
Watch for the color change in the solution. As the acid neutralizes the base, the solution will turn pink.
-
Stop adding the acid when the solution turns pink. This indicates the endpoint of the titration, where the acid and base have completely neutralized each other.
-
Record the final reading on the burette. Subtract the initial reading to find the volume of acid used.
-
Repeat the titration at least three times to obtain consistent readings.
Observations
| Trial Number | Initial Reading (mL) | Final Reading (mL) | Volume of Base Used (mL) |
|---|---|---|---|
| 1 | |||
| 2 | |||
| 3 |
Calculation
Using the formula for neutralization: \(N_{1}\times V_{1} = N_{2}\times V_{2}\)
Where:
-
\(N_{1} = Normality\ of\ base\ (NaOH)\)
-
\(V_{1} = Volume\ of\ base\ used\ (from\ the\ burette\ readings)\)
-
\(N_{2} = Normality\ of\ acid\ (HCl)\ (unknown)\)
-
\(V_{2} = Volume\ of\ acid\ (25\ mL)\)
Conclusion
The concentration of HCl is ________.
Reflect and Discuss
-
Why do you think it’s important to swirl the flask during the titration?
-
What do you think would happen if we took sodium hydroxide in the conical flask and hydrochloric acid in the burette instead? How would this change the way we detect the endpoint? Which of the two options do you prefer?