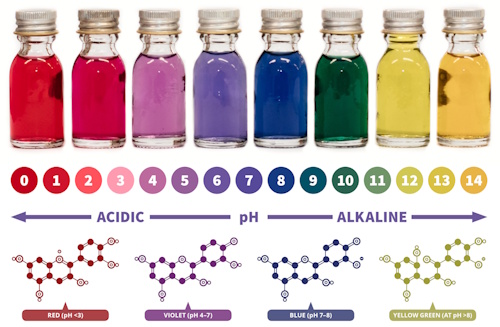
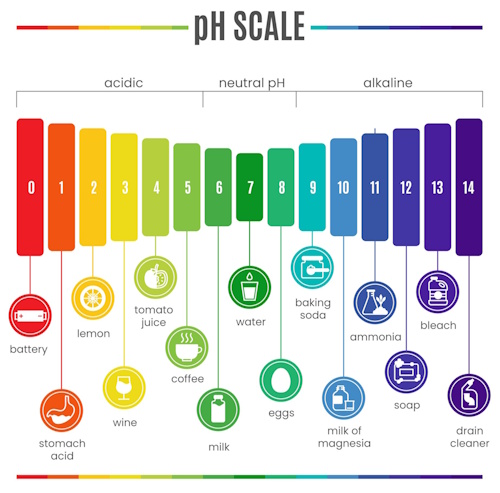
Have you ever noticed how a splash of lemon juice can change the color of certain foods? Or how turmeric stains become red when you add soap water? These color-changing reactions are not just kitchen surprises – they are tiny chemistry experiments!
In this activity, you will explore how natural materials like red cabbage, hibiscus, beetroot, and turmeric can reveal whether a substance is an acid or a base.