Certain food items like lemons, tamarind, and vinegar are sour to taste, while some substances like soap are slippery to touch and bitter in taste. It has been found that these items contain acids and bases. However, it is not safe to determine the nature of items by tasting or touching them. This is where indicators help us. Indicators are substances that change color in the presence of an acid or base, making it easier to identify their nature.
In this activity, you will explore some commonly used laboratory indicators and see how they help us identify the nature of different solutions.
Science Involved
Indicators are special substances that help us identify whether a solution is acidic, basic, or neutral by changing color. Common laboratory indicators include litmus, phenolphthalein, methyl orange, and the universal indicator.
-
Litmus is one of the oldest and simplest indicators. It turns red in acids and blue in bases but shows no color change in neutral solutions.
-
Phenolphthalein remains colorless in acids and turns pink in bases
-
Methyl orange changes from red in acids to yellow in bases, staying orange when neutral.
-
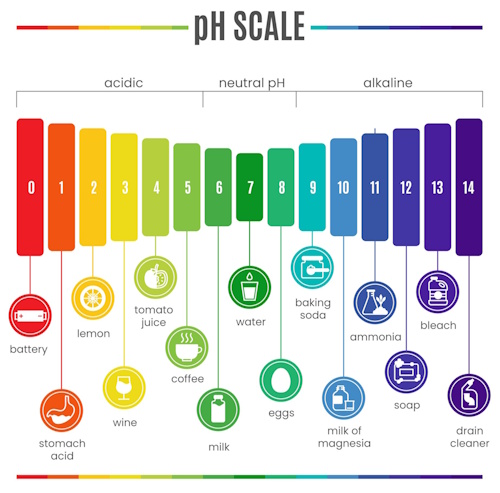
The universal indicator is different from the others because it is a mixture of several indicators. Instead of showing just two colors, it displays a range of colors depending on the pH of the solution. The pH scale runs from 0 to 14, where:
-
pH < 7 indicates an acid (stronger acids have lower pH values),
-
pH = 7 indicates a neutral solution, and
-
pH > 7 indicates a base (stronger bases have higher pH values).
-
The universal indicator thus gives a more detailed idea of how strong or weak an acid or base is – showing red for strong acids, orange to yellow for weak acids, green for neutral solutions, blue for weak bases, and violet for strong bases.
Activity
Requirements
Litmus paper (red and blue), phenolphthalein solution, methyl orange solution, Universal indicator solution, test tubes and test tube stand, droppers, common acidic substances (e.g., vinegar, lemon juice) and common acids (dilute hydrochloric acid or dilute sulfuric acid), common basic substances (e.g., baking soda solution) and sodium hydroxide solution and neutral substances (e.g., distilled water, ethyl alcohol).
Safety Instructions
You’ll be working with acids and bases that can irritate your skin or eyes. Always wear safety goggles and a lab coat. Handle acids and bases carefully and never taste or touch them. If you spill any chemical, wash the area immediately with plenty of water and inform your teacher or parents.
Procedure
-
Label the test tubes for the different substances you will test.
-
Pour about 2–3 mL of each substance into separate, clean test tubes.
-
Using droppers, test each substance with the following indicators:
-
Litmus paper: Dip both red and blue litmus papers into the test solution and note any color change.
-
Phenolphthalein: Add 2–3 drops of phenolphthalein solution and observe the color.
-
Methyl orange: Add 2–3 drops of methyl orange solution and record the color.
-
Universal indicator: Add 2–3 drops of universal indicator solution and compare the resulting color with the pH color chart to determine the approximate pH value.
-
-
Record your observations carefully.
Observations
| Test Solution | Litmus Paper (Red/Blue) |
Phenolphthalein | Methyl Orange | Universal Indicator |
|---|---|---|---|---|
| Hydrochloric acid | ||||
| Vinegar (acetic acid) | ||||
| Lemon juice | ||||
| Soda water or clear carbonated drink | ||||
| Baking soda solution | ||||
| Sodium hydroxide solution | ||||
| Distilled water |
Reflect and Discuss
-
Why do you think different indicators (like litmus, phenolphthalein, and methyl orange) show different color changes for the same acid or base?
-
If you had to design a new laboratory indicator, what properties would you want it to have?
