A molecule is so small that millions of them can fit into the head of a pin! Scientists in the early 20th century used clever experiments to figure out how big molecules are. In this activity, you will create an extremely thin layer of oil on water and use simple measurements to get an estimate of the size of a single molecule. It’s a little bit of math, a little bit of observation, and a lot of fun detective work in science.
Science Involved
Oil molecules are like tiny matchsticks with two different ends. One end is hydrophilic (water-loving) and wants to stay in contact with water, while the other end is hydrophobic (water-fearing) and wants to avoid water.
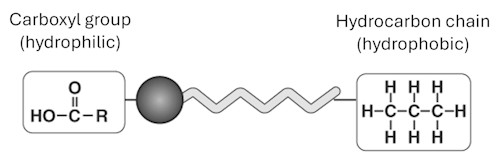
When the oil is dropped onto water, the hydrophilic ends point down into the water and the hydrophobic ends stick up into the air. This makes the molecules stand neatly side by side in a sheet that is just one molecule thick. By measuring how far the oil spreads, you will be able to estimate the size of a single molecule – an amazing way to peek into the invisible world!
We need to use only a very small amount of oil because if we used more, the oil would spread into a very large circle, and we would need a plate bigger than what we have. To manage this, we dilute the oil using alcohol. Alcohol helps because it mixes with both oil and water, allowing us to release just a tiny amount of oil onto the water’s surface.
Activity
Requirements
Shallow tray or plate of water, cooking oil (such as coconut oil, sunflower oil, or olive oil), alcohol (spirit or sanitizer containing alcohol), measuring cylinder or syringe, dropper, talcum powder and a ruler.
Getting Ready
Prepare a Dilute Oil Solution
-
To prepare a dilute oil solution measure 1 ml of cooking oil and mix it with 9 ml of alcohol. Shake or stir gently so it mixes well.
-
Take 1 ml of this solution and add it to 9 ml of alcohol again. Mix well.
-
Repeat once more by taking 1 ml from this second solution and adding it to 9 ml of alcohol. After three such steps, you now have 1 part in 1000 dilution of oil.
Procedure
-
Fill a shallow tray or plate with clean water. Sprinkle a light layer of talcum powder evenly over the water surface. This will help you see where the oil spreads.
Tray of water with fine powder sprayed on top. Image Credit: "Molecules in Motion". Practical Physics Collection, Institute of Physics. -
Using a dropper, take 1 drop of the diluted oil solution and carefully place it in the center of the water surface.
-
Watch closely as the oil spreads out into a very thin circular layer, pushing the powder to the sides. Measure the diameter of this circle using a ruler. Note it down.
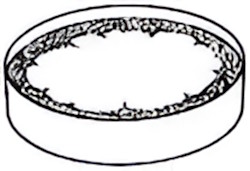
Powder displaced to side by the layer of oil. Image Credit: "Molecules in Motion". Practical Physics Collection, Institute of Physics. -
Repeat this step 2–3 times and record the diameter each time to get an average. Make sure that you clean the plate properly each time.
-
To calculate the volume of one drop, count how many drops from the dropper fill 1 ml of water, and then divide 1 ml by the number of drops. For example, if 20 drops make 1 ml, then 1 drop has a volume of 0.05 ml.
-
Now calculate the thickness of the oil layer. The thickness is equal to the volume of oil in one drop divided by the area of the circle it formed.
-
The thickness you calculate will give you an estimate of the size of an oil molecule, since in the thin layer, the oil molecules line up roughly one molecule thick!
-
Record your results and compare them with your initial guess or hypothesis about how small you thought a molecule might be.
Observations
Volume of 1 drop of solution: _____ ml
Volume of oil in 1 drop of solution (V): _____ ml
Radius of the oil film:
|
Reading 1 |
Reading 2 |
Reading 3 |
Average |
|---|---|---|---|
Thickness (height) of the oil film \(\left( h = \frac{V}{\pi r^{2}} \right)\): _____ cm
Number of carbon atoms forming the chain in the oil that you used: _____
Reflect and Discuss
-
Would your result change if you used a different oil?
-
Assuming that atoms of carbon are next to each other in the molecule, estimate the size of the carbon atom. Compare the result you obtain with the actual size of the carbon atom (about \(1.5 \times 10^{-10} m\)).
