A candle holds secrets of chemistry, physics, and even the composition of air! Michael Faraday, one of the greatest scientists of his time, once gave a famous lecture series called The Chemical History of a Candle, where he explored how a simple flame reveals amazing scientific principles.
In this activity you will practice the scientific method by observing the candle and its flame under different conditions. As aspiring scientists, your most important tool right now is your observational ability. This activity is primarily about honing that skill. Since you're learning the scientific method, don't worry about getting all the "right" answers! Many of the explanations for what you observe are complex and will make more sense as you master higher-level science. For now, focus on seeing, recording, and questioning. Get ready to investigate this everyday object with new eyes – there’s a lot more science hidden in a candle than you might think!
Activity
Requirements
Candles, matches or lighter, a heat safe plate with a raised edge, glass bell jar or bottle with a wide mouth and a couple of inches taller than the candles and water.
Safety Instructions
Always ask an adult for help when working the candle. Never leave heat sources unattended and handle them carefully to avoid burns. Keep water nearby just in case.
Procedure
Part 1: Fire

-
Place the candle on a plate and light it. From the moment you start, make note of everything you see – right from the candle's appearance before lighting it to what happens as it burns. Pay attention to any changes and details you notice and record your observations.
-
For each observation, propose a possible explanation (hypothesis) for what you think is happening with the candle as it burns.
Part 2: Air
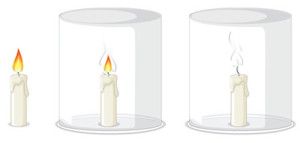
-
Carefully cover the lit candle with the inverted glass jar. Observe closely and note down everything you see. Pay attention to any changes in the flame, the candle, or the surrounding environment.
-
Remove the jar, let the candle cool, and, if necessary, repeat the steps a couple of times if required to confirm your observations.
-
Based on your observations, propose a possible explanation for why the flame behaves the way it does under the glass jar.
-
Think of an additional experiment to test the hypothesis. Before performing the experiment, write down what you expect will happen based on your hypothesis. What outcome would support your hypothesis, and what would challenge it?
-
Perform the experiment to test your hypothesis. Record the results and compare them to your expected outcome. Do the results confirm your hypothesis, or do you need to modify it?
Part 3: Water
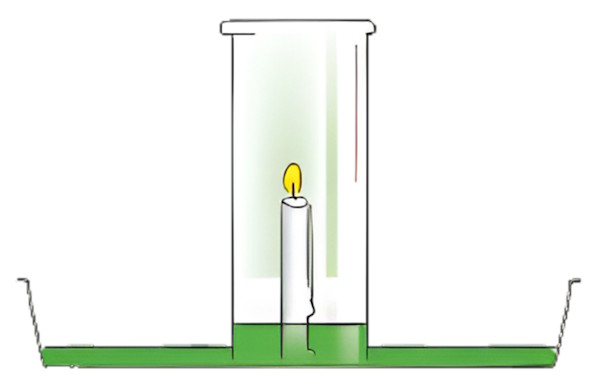
-
Place a small amount of water on the plate with the candle and light the candle. Then, cover the lit candle with the inverted glass jar. Observe and record everything that happens.
-
Based on your observations, propose an explanation for what happened.
-
Perform another experiment to verify your hypothesis.
Reflect and Discuss
-
Were your initial hypotheses correct?
-
How did your understanding change after each experiment?
References and Further Reading
- Faraday, Michael. The Chemical History of a Candle, Chatto & Windus. 1908.
- Hammack, Bill and DeCoste, Don. Michael Faraday’s The Chemical History of a Candle with Guides to Lectures, Teaching Guides & Student Activities, Articulate Noise Books. 2016.

