Potato cells contain water along with dissolved minerals and salts that help it stay firm and healthy. When a potato is placed in different surrounding solutions, water moves in or out of its cells depending on how concentrated those solutions are. This movement of water reveals important information about the internal environment of the potato.
In this activity, you will estimate the approximate concentration of the solution inside a potato using the process of osmosis. By placing potato strips in solutions of different concentrations and measuring how their mass changes, we can determine the point at which there is no net movement of water. That concentration will be closest to the natural salt and mineral concentration within the potato cells.
Science Involved
Osmosis is the movement of water across a semi-permeable membrane from a region of lower solute concentration to a region of higher solute concentration. In plant cells, osmosis plays a crucial role in maintaining cell structure and function. When placed in different solutions, plant cells either gain or lose water depending on the concentration of solutes in their environment.
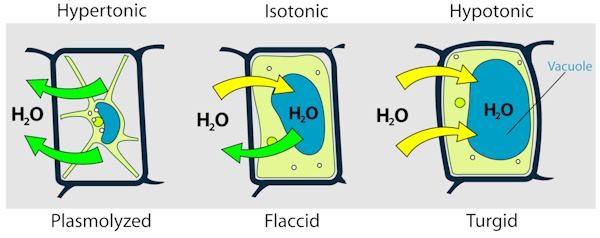
-
Isotonic solution: The concentration of solutes is the same inside and outside the cell, so there is no net movement of water.
-
Hypotonic solution: The surrounding solution has a lower solute concentration than the cell’s interior, causing water to enter the cell and making it swell.
-
Hypertonic solution: The surrounding solution has a higher solute concentration than the cell’s interior, causing water to leave the cell and making it shrink.
Osmosis explains many everyday situations – for example, why we feel thirsty after eating salty food, why vegetables turn limp when sprinkled with salt, and why ORS (Oral Rehydration Solution) must contain the right balance of salt and sugar to restore body fluids. Even medical processes like dialysis depend on maintaining the correct concentration of solutes so that water moves safely across membranes.
Activity
Requirements
1 large potato, sugar solutions of different concentrations: 0%, 2%, 4%, 6%, 8%, 10% (by mass), 6 labelled test tubes or beakers, digital balance, knife, paper towels or tissue paper.
Procedure
-
Label the 6 test tubes or beakers with the salt concentrations: 0%, 2%, 4%, 6%, 8%, and 10%.
-
Pour 10 mL of the corresponding salt solution into each test tube.
-
Cut uniform samples of potato (about 3 cm in length and similar width). Do not include any skin.
-
Blot the potato sample with a paper towel to remove excess moisture.
-
Weigh each potato sample and record its initial mass.
-
Place one potato sample into each test tube.

-
Allow the potato sample to sit in the solutions for 15-20 minutes.
-
After the waiting time, remove the potato sample using forceps and blot them gently with a paper towel.
-
Weigh each potato sample again and record the final mass.
-
Calculate the percentage change in mass using the formula: $$Percentage\ Change = \frac{Final\ Mass - Initial\ Mass}{Initial\ Mass} \times 100$$
-
Plot a graph of the percentage change in mass vs concentration of sugar solution.
Observations
| Sugar Concentration (%) | Initial Mass (g) | Final Mass (g) | Percentage Change (%) |
|---|---|---|---|
| 0% (Distilled Water) | |||
| 2% | |||
| 4% | |||
| 6% | |||
| 8% | |||
| 10% |
Reflect and Discuss
-
What would your results be if the potato were placed in a dry area for several days before your experiment?
-
When potatoes are in the ground, do they swell with water when it rains?
-
How does storing fruits and vegetables in pure water or saltwater affect their texture?
-
Why must the concentration of salts and glucose in an ORS (Oral Rehydration Solution) be carefully balanced? What might happen if the solution is too concentrated or too dilute?
References
-
"Diffusion and Osmosis". AP Biology Investigative Labs. The College Board New York. 2012.
-
"Potato Osmosis Lab". DataClassroom. Accessed November 3, 2025.
